撫平面部皺褶紋理 隱藏肌齡秘密
Anti-wrinkle & anti-aging
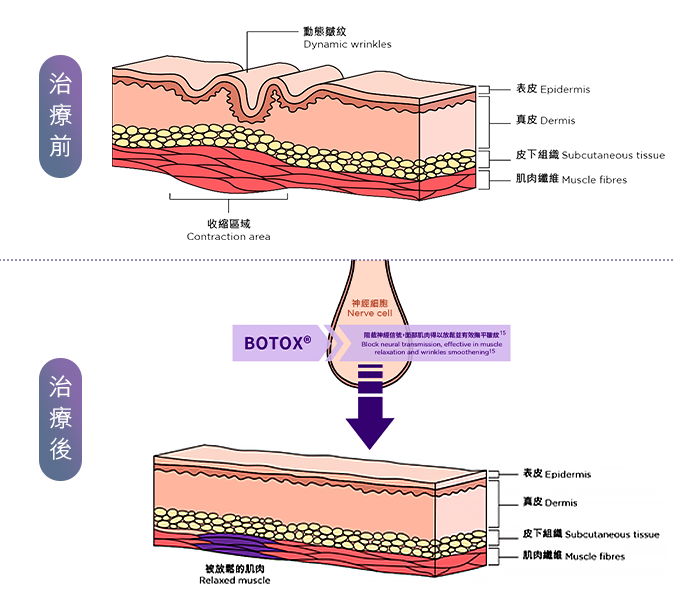
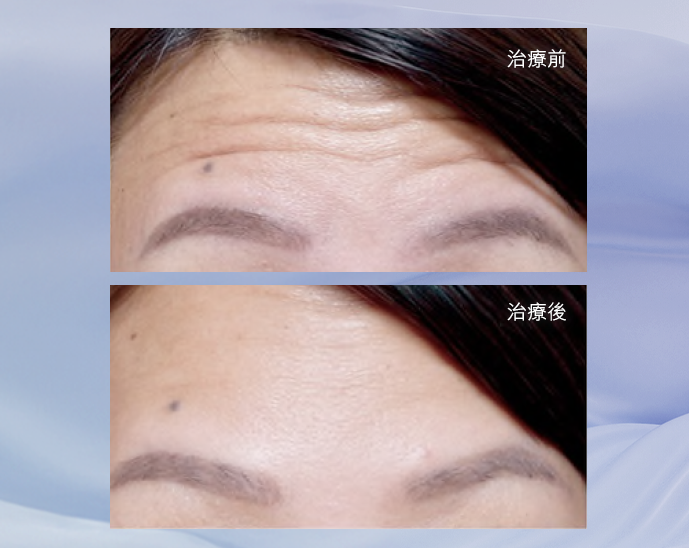
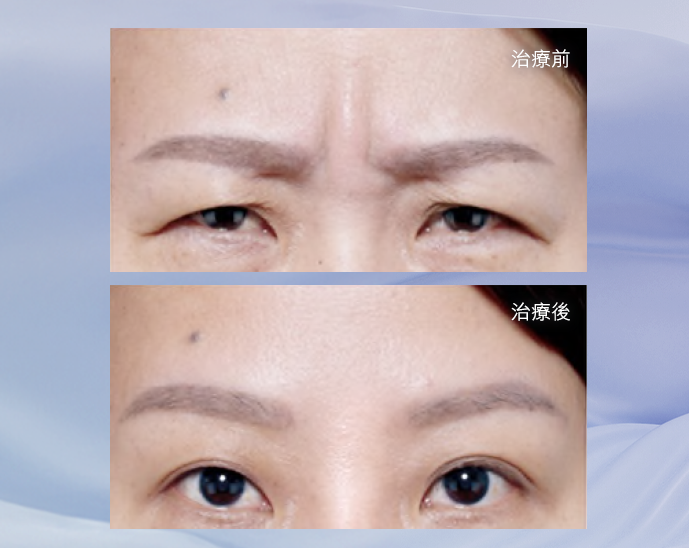
BOTOX®保妥適®肉毒桿菌素專為撫平歲月累積的表情紋而設, 為全球銷量No.1品牌,目前已超過1億瓶銷售,並榮獲美國FDA認證超過30年,同時超過4,200份醫學文獻及研究支持。 當肌膚注入經高科技純化、肉毒桿菌A型中萃取的蛋白質的瞬間,時間在臉上仿如凝止,年輕永駐之旅從此揭開序幕。 只需十分鐘,BOTOX®保妥適®肉毒桿菌素能降低肌肉收縮張力,有效放鬆導致動態皺紋的面部肌肉,臉上各種曾被歲月刻劃過的細紋、皺紋,均被撫平、一一拭去。
效果見證
選擇BOTOX®保妥適®肉毒桿菌素療程的原因
美國FDA認證超過30年,安全有效
全球銷量超過 1 億瓶,信心保證
FDA 認證撫平三大皺紋
去皺效果⻑達6個⽉
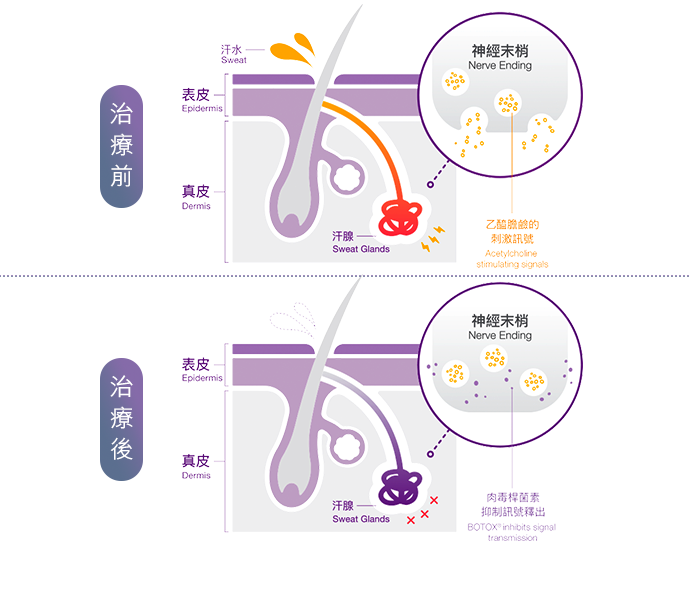
原理及技術
FAQ
*療程效果因人而異,以上資料和相片由廠商提供,只供參考*
*療程前應根據實際情況,咨詢及參考專業意見*
*COLLAGEN+保留隨時更改或終止條款及細則的權利,而無須另行通知*
*如有任何爭議,COLLAGEN+保留最終決定權*
References:
Medical Insight, Inc 2018. Global Facial Injectables Market Study. December 2018. Based on worldwide neurotoxins market 2017 (in sales value).
Allergan. Unpublished Data. INT/0292/2018(1). March 2019. #: BOTOX® Prescribing Information. HK-41906. May 2013.
Allergan. Data on File. REF-74184.UNPUBLISHED DATA: BOTOX® number of vials shipped as of March 2019 INT/0292/2018(1). March 2019.
1. Trindade de Almeida A et al. Dermatol Surg 2015; 41: S19-S28.
2. Yu W et al. J Cosmet Laser Ther. 2018Oct;20(5):278-286.
3. U.S. Food and Drug Administration, Department of Health & Human Services. Botox approval letter 85-0226 & 85-0227, December 29, 1989.
4. Allergan. Unpublished Data. INT-BCT-2050129 – Worldwide BOTOX® marketing authorization status. October 2020.
5. Allergan. Unpublished Data. INT-BCT-2050035. BOTOX® vs competitors – number of peer reviewed articles vs competitors. February 2020.
6. Flaharty, P. (2014, April 8). Your facial wrinkles are dynamic or static. Retrieved February 10, 2022, from https://www.news-press.com/story/life/wellness/2014/04/08/beauty-tips-your-facial-wrinkles-are-dynamic-or-static/7432785/.
7. DasGupta BR. Structures of botulinum neurotoxin, its functional domains, and perspectives on the crystalline type A toxin. In: Jankovic J, Hallet, M., ed. Therapy with Botulinum Toxin. New York, NY: Marcel Decker, Inc; 1994: 15-39.
8. Coffield JA, Considine RV, Simpson LL. The site and mechanism of action of botulinum neurotoxin. In: Jankovic J, Hallet, M., ed. Therapy with Botulinum Toxin. New York, NY: Marcel Dekker, Inc; 1994:3-13.
9. Schantz EJ, Johnson EA. Preparation and characterization of botulinum toxin type A for human treatment. In: Jankovic J, Hallet, M., ed. Therapy with Botulinum Toxin. New York, NY: Marcel Dekker, Inc; 1994:41-49.
10. Simpson LL. The actions of clostridial toxins on storage and release of neurotransmitters. In: Harvey AL, ed. Natural and Synthetic Neurotoxins (Neuroscience Perspectives). San Diego, CA: Academic Press; 1993:278-317.
11. Black JD, Dolly JO. Interaction of 125I-labeled botulinum neurotoxins with nerve terminals II. Autoradiographic evidence for its uptake into motor nerves by acceptor-mediated endocytosis. J Cell Biol. 1986;103(2):535-544.
12. Lebeda FJ, Hack DC, Bentry MK. Theoretical analyses of the functional regions of the heavy chain of botulinum neurotoxin. In: Jankovic J, Hallet M, ed. Therapy with Botulinum Toxin. New York, NY: Marcel Dekker, Inc; 1994:51-61.
13. Harvey AL. Presynaptic toxins. In: Smythies JR, Bradley RJ, ed. International Review of.
14. Neurobiology. San Diego, CA: Academic Press; 1990:201-239.
15. Tremaine AM, McCullough JL. Clin Cosmet Investig Dermatol. 2010;3:15–23.
16. Stotland MA et al. Plast Reconstr Surg 2007; 120(5):1386-9.
17. Beer KR et al. J Drugs Dermatol 2011;10:39-41.
18. De Boulle K et al. Dermatol Surg. 2018;44[11]:1437-1448.
19. Hyperhidrosis- Causes and Treatment of Enhanced Sweating. Dtsch Arztebl Int. 2009 Jan; 106(3):32-37.
20. Aesthetic Surg J. 2012 Feb;32(2):238-244.
21. J Am Acad Dermatol. 2007 Apr; 56(4):604-11.
22. He J, Wang T and Dong J. Journal of Dermatological Treatment. 2012 23: 461–464.
23. Naumann M and Lowe NJ. BMJ 2001;323:1–4.
4. Lowe NJ et al. J Am Acad Dermatol. 2007;56(4):604-611. 25. U.S. Food and Drug Administration, Department of Health & Human Services. Botox approval letter SUPPL-5303, October 2, 2017.
BOTOX®保妥適®(Botulinum Toxin Type A)藥品註冊編號: HK-41906. BOTOX®為醫生處方藥物,請向醫生或醫學美容中心
諮詢切合您獨特需要的專屬療程方案。療程效果因人而異。療程後如有任何不良反應,請即向醫生查詢。













-1751511351.png)