一次長效肌底補濕 逆轉光澤水潤肌膚
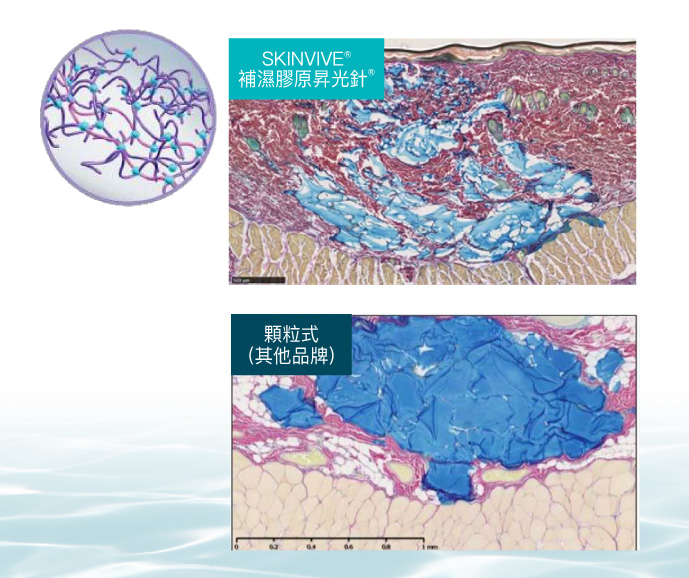
Skinvive® 補濕膠原昇光針為全球第一及唯一獲得美國FDA及歐盟CE雙重認證的透明質酸水光品牌,透過Juvéderm®的國際專利VYCROSS™交聯鏈結科技,混和長短鏈透明質酸,達至絕佳的肌膚組織融合度。
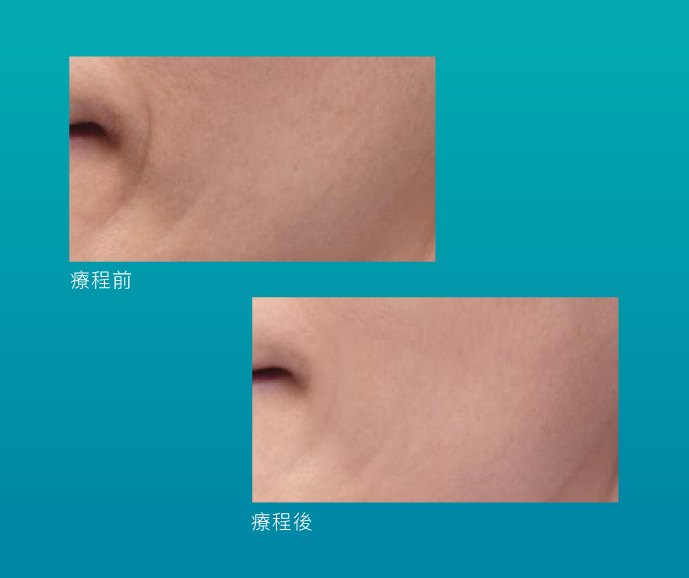
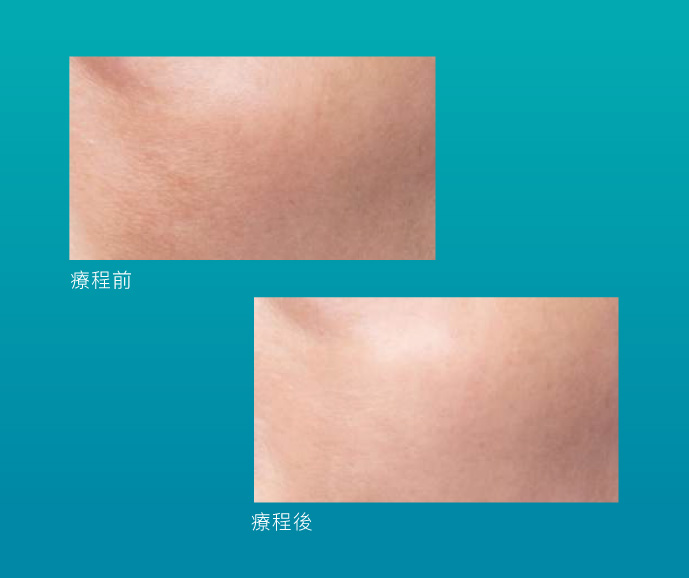
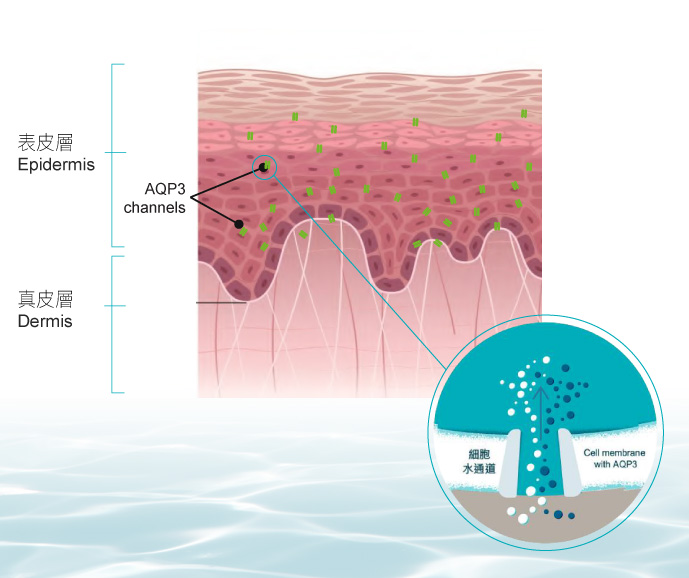
Skinvive®質感輕薄,能夠輕鬆深透至真皮深層,增加及提升細胞水通道AQP3,有助表層細胞從底層吸收更多水分和甘油,由內而外滋潤肌膚。同時建立優質微環境,促進膠原蛋白自生,恢復肌膚屏障及彈性活力。Skinvive®單次療程能夠締造長達9個月的自然持久效果,肌底長效補濕的同時,高效逆轉膚質及光澤感,煥發健康肌膚。
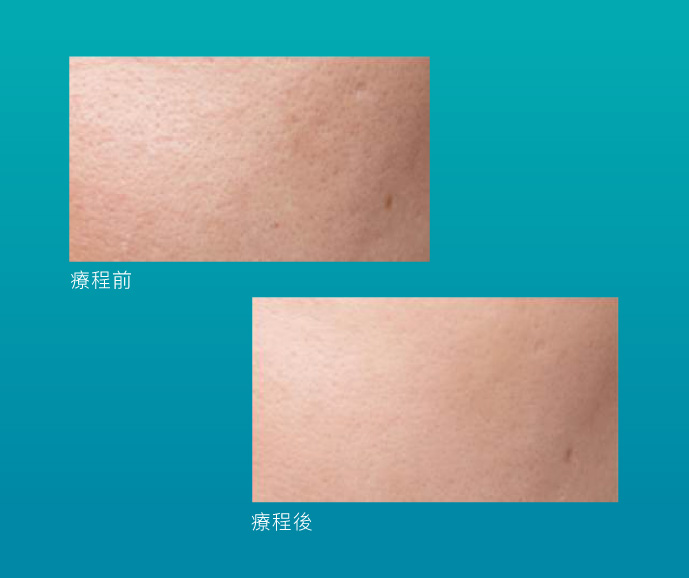
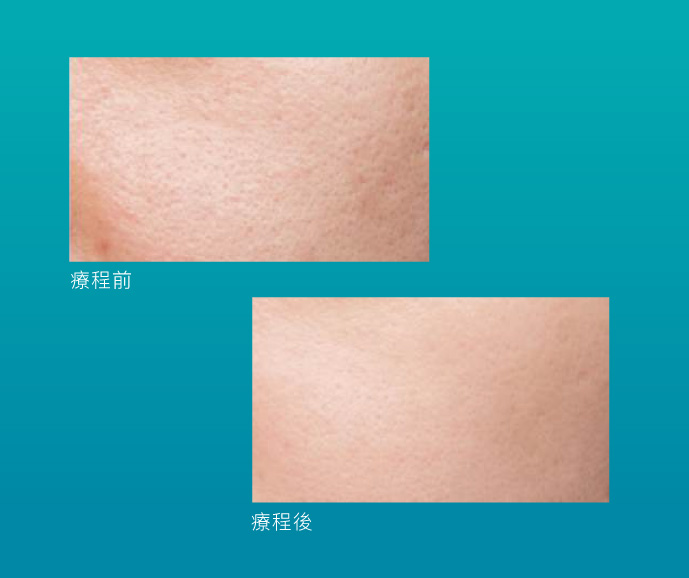
效果見證
選擇Skinvive® 補濕膠原昇光針的原因
全球第一及唯一獲得美國FDA及歐盟CE雙重認證的透明質酸水光品牌
國際專利VYCROSS™ 交聯鏈結科技,達至絕佳的肌膚組織融合度,療程效果持久自然
唯一高隱定性透明質酸水光療程,單次療程帶來長達9個月的深層水潤效果
直接釋放水分至真皮層,提升細胞水通道,由深至淺長效補水滋潤皮膚
有別於其他水光療程只集中於強效補水,Skinvive 同時能夠改善膚質平滑度,提升3倍光澤滿意度
91%用家滿意膚質改善效果,97%用家在第二次療程後願意向朋友推薦
內含利多卡因減痛成份,令療程更舒適放心
原理及技術
FAQ
*療程效果因人而異,以上資料和相片由廠商提供,只供參考*
*療程前應根據實際情況,諮詢及參考專業意見*
*COLLAGEN+保留隨時更改或終止條款及細則的權利,而無須另行通知*
*如有任何爭議,COLLAGEN+保留最終決定權*
Footnotes:
* Skin hydration was measured using the MoistureMeterD instrument with the XS 5 and S 15 probes (depth of effective measurement: 0.5 and 1.5 mm, respectively).4
^ Based on a search of published clinical studies of injectable HA treatments in March 2023. A duration of 9 months of skin hydration was established with SKINVIVETM.8
† Instrument measures of skin hydration, smoothness, and skin deformation parameters were performed on the cheek, forehead, and neck of one side of the face on Day 0 and at 30 days after initial treatment (before touch up), Months 1, 4, 6, and 9 after the last treatment, and Month 1R. Skin hydration was measured using the MoistureMeterD instrument (n=131) and top-up treatment administered at Day 30 (n=31).4
‡ Lasting up to 9 months as reported by 64.6% of patients. As reported by patients (127/131) in a composite satisfaction score, which includes how clear their facial skin looked.5
§ Lasting up to 9 months as reported by 63% of patients. As reported by patients (127/131) in a composite satisfaction score, which includes how radiant their facial skin looked.5
‖ Lasting up to 9 months as reported by 55.1% of patients. As reported by patients (127/131) in a composite satisfaction score, which includes how smooth their facial skin looked.5
¶ Lasting up to 9 months as reported by 67.7% of patients. As reported by patients (127/131; 3 subjects did not respond) in a composite satisfaction score, which includes satisfaction with their skin’s tone (colour).5
** Lasting up to 9 months as reported by 55.9% of patients. As reported by patients (127/131) in a composite satisfaction score, which includes how their pores looked.5
‡‡ Data derived from a prospective, non-randomised, interventional, open-label study where 11 healthy patients (men [n=2] and women [n=9]) received intradermal VYC-12L treatment on the volar forearm. Clinical probes assessed skin quality at baseline and Months 1 and 3 post-treatment. Punch biopsies were collected at Months 1 and 3 post-treatment to evaluate histologic and genomic changes.13
§§ The primary effectiveness endpoint, the percent‐ age of subjects’ cheeks with ≥1‐point improvement from baseline on the Allergan Skin Roughness Scale (ASRS ) ,6 was achieved in 96 .2 % of cheeks at month.5
‖‖ Subjects completed the FACE-Q Satisfaction With Skin scale at baseline (Day 0, before treatment), Months 1, 4, 6, and 9 (before repeat treatment, if received), and Month 1 after the repeat treatment (n=131; n=62 received repeat treatment). The Satisfaction With Skin scale is a validated patient-reported outcome instrument designed to assess the impact of treatment from the subject’s perspective. The scale comprises 12 questions about satisfaction with different facial skin attributes (e.g. smoothness, clearness, radiance). Responses are rated on a 4-point scale (1: very dissatisfied, 4: very satisfied). Scores for each item were summed and converted to a scale score (range: 0–100; higher scores indicate greater satisfaction) using the algorithm developed by the FACE-Q developers.5
¶¶ 97% of subjects said they would recommend treatment to a friend after repeat treatment at month 1.5
*** The Fitzpatrick classification denotes six different skin types, skin colour and reaction to sun exposure (type I: burns easily, never tans; type II: burns easily, tans minimally with difficulty; type III: burns moderately, tans moderately and uniformly; type IV: burns minimally, tans moderately and easily; type V: rarely burns, tans profusely; type VI: never burns, tans profusely).6 In a randomised, evaluator-blind, delayed treatment control study involving 209 patients, the majority of patients had Fitzpatrick skin types III/IV (58.8%), followed by I/II (32.0%) and V/VI (9.2%).2 In a prospective, single-arm study of 131 patients, the majority of patients had Fitzpatrick skin type III (53.4%), followed by II (35.1%), IV (10.7%) and V (0.8%).4
References:
1. Humphrey S et al. Dermatol Surg. 2021;47(7):974–981; 2. Fisher GJ et al. Arch Dermatol. 2008;144:666–672; 3. Walker M. Int J Pharm. 2022;622:121850; 4. Niforos F et al. Clin Cosmet Investig Dermatol. 2019;12:791–798; 5. Ogilvie P et al. J Cosmet Dermatol. 2020;19(5):1065–1070; 6. Belmontesi M et al. J Drugs Dermatol. 2018; 17(1): 83–88; 7. SKINVIVE® by Juvéderm® DFU. 20074348. Revision 2022.09.30; 8. Allergan Aesthetics. Unpublished data. SKINVIVE® by JUVÉDERM® – 9-month duration of skin hydration in clinical trials. March 2023; 9. Allergan Aesthetics. Unpublished data. JUVÉDERM®, the world’s leading brand of hyaluronic acid facial fillers. REF-108642. March 2023; 10. SKINVIVE® by Juvéderm® is FDA approved. U.S. Food and Drug Administration, Department of Health & Human Services. SKINVIVE by Juvéderm approval letter P110033/S059, May 2023; 11. SKINVIVE® by Juvéderm® is CE approved. European Commission, hyaluronic acid based medical devices. EC certificate number: 3824296CE05; 12. Allemann IB and Baumann L. Clin Interv Aging. 2008;3(4):629–34; 13. Safa M et al. Clin Cosmet Investig Dermatol. 2022;15:411–426; 14. Draelos Z. J Clin Aesthet Dermatol. 2012;5(7):53–56; 15. Kapoor KM et al. Clin Cosmet Investig Dermatol. 2021;14:1105–18; 16. Purnamawati S et al. Clin Med Res. 2017;15(3-4);75–87; 17. Alexiades M et al. Dermatol Surg. 2023;49(7):682–688; 18. Akinbiyi T et al. Plast Reconstr Surg Glob Open. 2020;8(10):e2763; 19. Lipko-Godlewska S et al. Clin Cosmet Investig Dermatol. 2021;14:169–178; 20. Zarbafian M et al. Dermatol Surg. 2022;48(3):369–372.